Frequently asked questions Frequently asked questions
There are extensive and exhaustive international regulations on the packaging and transport of samples. Ignorance of these regulations by the agents involved in these processes can lead to fines and sanctions and endanger the integrity of the people exposed to these substances.
In this section we will try to answer all your questions about these guidelines.
How do I know if a substance is considered infectious and in which category does it fall?
How are substances classified for transport?
For transport purposes, the WHO classifies class 6.2 infectious substances into three categories:
- Category A
- Category B
- Exempt human or animal sample
Category B was created by the ADR in its 2003 edition, in order to homogenise and economise shipments without breaching the regulations, and includes almost all transported substances. This change is due to the fact that grouping all substances in the same Category A entailed a very high logistical effort that was not commensurate with the risk of infection of some of the substances.
Most of the samples sent do not pose as high a risk as those included in Category A. However, just as during an ordinary hospital day any blood collection is carried out, the protocol is adequate for a contaminated sample (sterilised single-use materials to be destroyed afterwards), Category B involves a series of measures that limit the risk of infection to an extraordinary degree and are compatible with the daily work and budgetary limitations of the organisations that manage them. Thus, the existing risk can be addressed with relatively modest measures.
How are substances classified according to their transport?
In the case of transporting infectious substances, the container must comply with triple packaging, comprising the following layers:
- Primary container. An airtight container containing the sample. The container is wrapped in absorbent material sufficient to absorb all the fluid in case of rupture.
- Secondary container. A second watertight, durable container that encloses and protects the primary container(s). Several primary receptacles may be enclosed in a secondary container, but sufficient absorbent material must be used to absorb all of the fluid in the event of a rupture.
- Outer packaging.
Each element shall be assigned the following properties
- Internal pressure: This characteristic can be met by either the primary or the secondary container, with the precaution that if in the certification it is the primary that meets it, the supplier has the legal obligation to also supply the container with that primary, i.e. the complete packaging..
- Rigid: either the secondary or the outer packaging must be rigid, but not both.
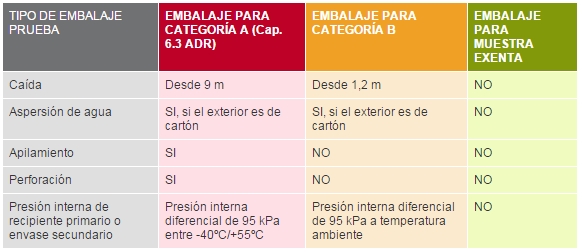
What tests must a container pass?
Containers must pass stringent endurance tests, including free-fall tests from a height of nine metres, puncture tests and pressure tests. The outer packaging must bear the UN standard packaging mark, which certifies that the packaging has passed the strength tests to the satisfaction of the competent authority. An official copy of the test report must be available.
And what packaging should category A samples be transported in?
According to the WHO Guidance on Infectious Substances Transport Regulations 2015/2016, Category A substances “are substances that are transported in a form which, when exposed to them, are capable of causing permanent incapacity, endangering life or constituting a fatal disease in previously healthy humans or animals”. This category includes substances such as Ebola virus, Masburg virus, Anthrax, Dengue virus, Mapucho virus, among many others.
Category A infectious materials may only be transported in packaging that meets the specifications of UN Class 6.2 and Packaging Instruction P620, ensuring that it has passed stringent resistance tests (free fall from 9 metres, puncture, pressure and stacking resistance). Importantly, the primary container or secondary packaging must withstand a pressure differential of not less than 95 kPa. The UN standard mark does not indicate that the packaging has been subjected to such tests, so users of the packaging should check with their suppliers whether the packaging meets this requirement.
-
The triple packaging system for the shipment of Category A infectious substances consists of three tiers:
- 1. Leakproof primary container: these may be sample tubes, petri dishes, urine bottles, etc. If it contains liquid samples, it is accompanied by an absorbent safety rack which, in the event of breakage, must retain all of the liquid being transported.
- 2. Secondary container: Allows the introduction of several primary containers with samples and can withstand an internal pressure of 95kPa in a temperature range between -40 ºC and +55 ºC.
- 3. Outer packaging: It includes stability elements to guarantee the correct position of the secondary containers and all the pictograms required for their transport. It must be resistant to water, traction, impact and chemical agents.