The management of biological risk in the preanalytical phase is based on maintaining the Chain of Biological Safety, which includes the prevention of risks, from the taking of samples to their final destruction as waste.
This entails adopting a strategic and integrated approach to the analysis and management of the risks of contaminants, for persons and the environment, and their adverse effects.

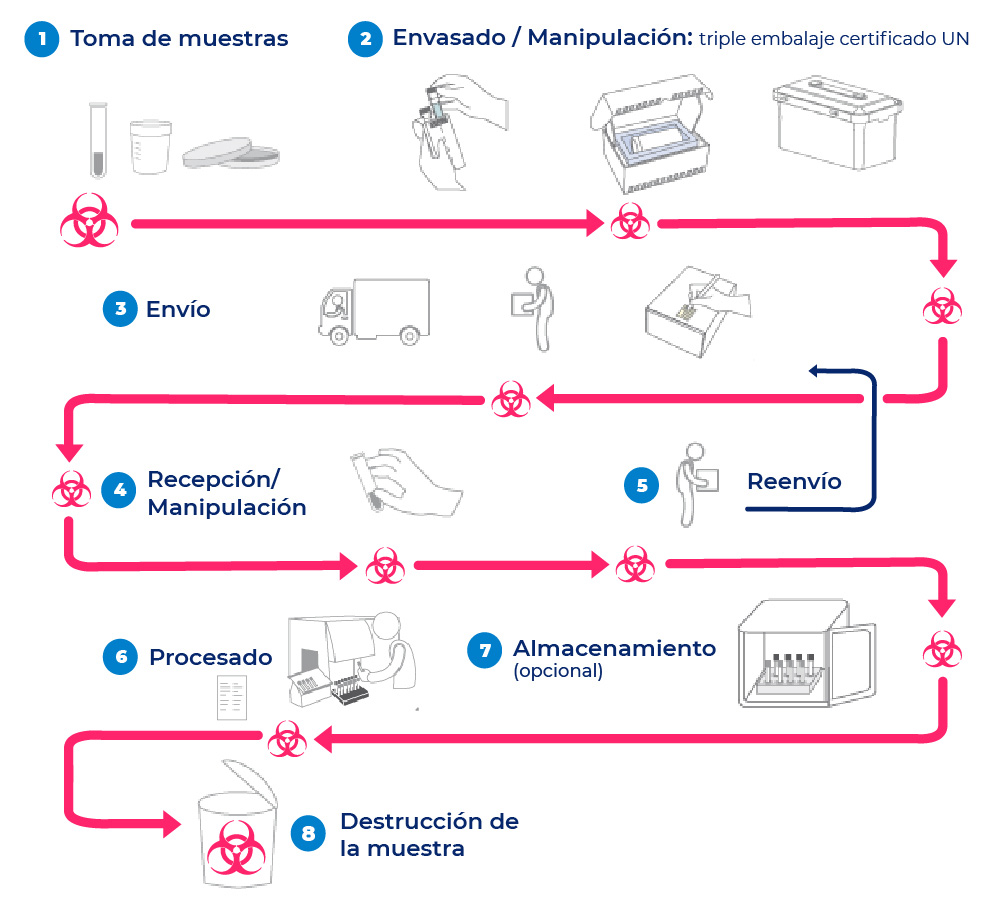
Keys for the correct approach to management
Before proceeding to package and transport a substance, it is essential to know whether it must be assigned to a certain category and treated in a specific way.
The WHO classifies infectious materials in three categories which will determine how they should be treated, and the logistics with respect to transportation.
The containers designed for the transportation of infectious substances must always comply with the Triple Packaging System recommended by the WHO.
Each container must pass strict resistance tests certified by the appropriate competent authority.
Category A infectious materials can only be transported in packaging/containers which have passed strict resistance tests.
The different regulations are designed to protect the environment and the persons involved in the handling, packaging and transportation of hazardous biological substances.
